Community Ecology
Section Goals
By the end of this section, you will be able to do the following:
- Discuss the predator-prey cycle
- Give examples of defenses against predation and herbivory
- Define the competitive exclusion principle
- Compare and contrast the different types of symbiotic relationships between species
Populations rarely, if ever, live in isolation from populations of other species. In most cases, numerous species share a habitat. The interactions between these populations play a major role in regulating population growth and abundance. All populations occupying the same habitat form a community: populations inhabiting a specific area at the same time. The number of species occupying the same habitat and their relative abundance is known as species diversity. Areas with low diversity, such as the glaciers of Antarctica, still contain a wide variety of living things. In contrast, the diversity of tropical rainforests is so great that it cannot be counted. Ecology is studied at the community level to understand how species interact with each other and compete for the same resources.
Predation and Herbivory Perhaps the classical example of species interaction is predation: the consumption of prey by its predator. Nature shows on television highlight the drama of one living organism killing another. Populations of predators and prey in a community are not constant over time; in most cases, they vary in cycles that appear to be related. The most often cited example of predator-prey dynamics is seen in the cycling of the lynx (predator) and the snowshoe hare (prey), using nearly 200-year-old trapping data from North American forests (Figure 1). This cycle of predator and prey lasts approximately ten years, with the predator population lagging 1–2 years behind that of the prey population. As the hare numbers increase, there is more food available for the lynx, allowing the lynx population to increase as well. When the lynx population grows to a threshold level, however, they kill so many hares that the hare population begins to decline, followed by a decline in the lynx population because of scarcity of food. When the lynx population is low, the hare population size begins to increase due, at least in part, to low predation pressure, starting the cycle anew.
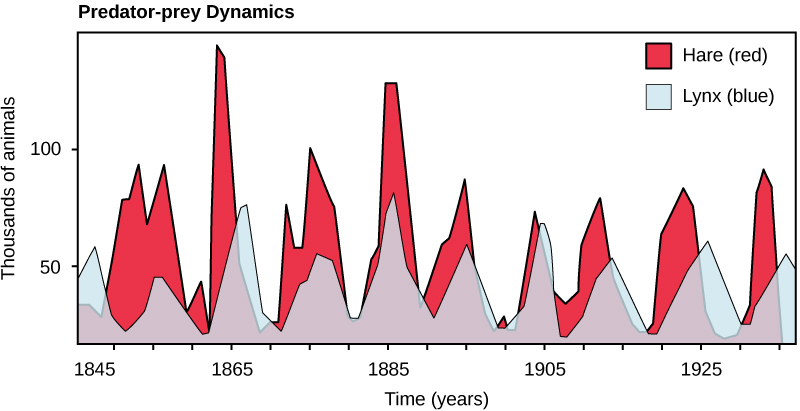
Some researchers question the idea that predation models entirely control the population cycling of the two species. More recent studies have pointed to undefined density-dependent factors as being important in cycling, in addition to predation. One possibility is that cycling is inherent in the hare population due to density-dependent effects such as lower fecundity (maternal stress) caused by crowding when the hare population gets too dense. The hare cycling would then induce the cycling of the lynx because it is the lynxes’ major food source. The more we study communities, the more complexities we find, allowing ecologists to derive more accurate and sophisticated models of population dynamics.
Herbivory describes the consumption of plants by insects and other animals, and it is another interspecific relationship that affects populations. Unlike animals, most plants cannot outrun predators or use mimicry to hide from hungry animals. Some plants have developed mechanisms to defend against herbivory. Other species have developed mutualistic relationships; for example, herbivory provides a mechanism of seed distribution that aids in plant reproduction.
Defense Mechanisms against Predation and Herbivory
The study of communities must consider evolutionary forces that act on the members of the various populations contained within it. Species are not static but slowly changing and adapting to their environment by natural selection and other evolutionary forces. Species have evolved numerous mechanisms to escape predation and herbivory. These defenses may be mechanical, chemical, physical, or behavioral.
Mechanical defenses, such as the presence of thorns on plants or the hard shells on turtles, discourage animal predation and herbivory by causing physical pain to the predator or by physically preventing the predator from being able to eat the prey. Chemical defenses are produced by many animals as well as plants, such as the foxglove, which is extremely toxic when eaten. Figure 2 shows some organisms’ defenses against predation and herbivory.

Many species use physical appearance, such as body shape and coloration, to avoid being detected by predators. The tropical walking stick is an insect with the coloration and body shape of a twig, which makes it very hard to see when stationary against a background of real twigs (Figure 3a). In another example, the chameleon can, within limitations, change its color to match its surroundings (Figure 3b). Both of these are examples of camouflage or avoiding detection by blending in with the background. There are many behavioral adaptations to avoid or confuse predators. Playing dead and traveling in large groups, like schools of fish or flocks of birds, are both behaviors that reduce the risk of being eaten.

Some species use coloration as a way of warning predators that they are not good to eat. For example, the cinnabar moth caterpillar, the fire-bellied toad, and many species of beetle have bright colors that warn of a foul taste, the presence of toxic chemicals, and the ability to sting or bite, respectively. Predators that ignore this coloration and eat the organisms will experience their unpleasant taste or the presence of toxic chemicals and learn not to eat them in the future. This type of defensive mechanism is called aposematic coloration or warning coloration (Figure 4).

While some predators learn to avoid eating certain potential prey because of their coloration, other species have evolved mechanisms to mimic this coloration to avoid being eaten, even though they themselves may not be unpleasant to eat or contain toxic chemicals. In Batesian mimicry, a harmless species imitates the warning coloration of a harmful one. Assuming they share the same predators, this coloration then protects the harmless ones, even though they do not have the same level of physical or chemical defenses against predation as the organisms they mimic. Many insect species mimic the coloration of wasps or bees, which are stinging, venomous insects, thereby discouraging predation (Figure 5).

In Müllerian mimicry, multiple species share the same warning coloration, but all of them actually have defenses. Figure 6 shows a variety of foul-tasting butterflies with similar coloration. In Emsleyan/Mertensian mimicry, a deadly prey mimics a less dangerous one, such as the venomous coral snake mimicking the nonvenomous milk snake. This type of mimicry is extremely rare and more difficult to understand than the previous two types. For this type of mimicry to work, it is essential that eating the milk snake has unpleasant but not fatal consequences. Then, these predators learn not to eat snakes with this coloration, protecting the coral snake as well. If the snake were fatal to the predator, there would be no opportunity for the predator to learn not to eat it, and the benefit for the less toxic species would disappear.

Did I Get It?
Competitive Exclusion Principle
Resources are often limited within a habitat, and multiple species may compete to obtain them. All species have an ecological niche in the ecosystem, which describes how they acquire the resources they need and how they interact with other species in the community. The competitive exclusion principle states that two species cannot occupy the same niche in a habitat. In other words, different species cannot coexist in a community if they are competing for all the same resources. An example of this principle is shown in Figure 7, with two protozoan species, Paramecium aurelia and Paramecium caudatum. When grown individually in the laboratory, they both thrive. But when they are placed together in the same test tube (habitat), P. aurelia outcompetes P. caudatum for food, leading to the latter’s eventual extinction.
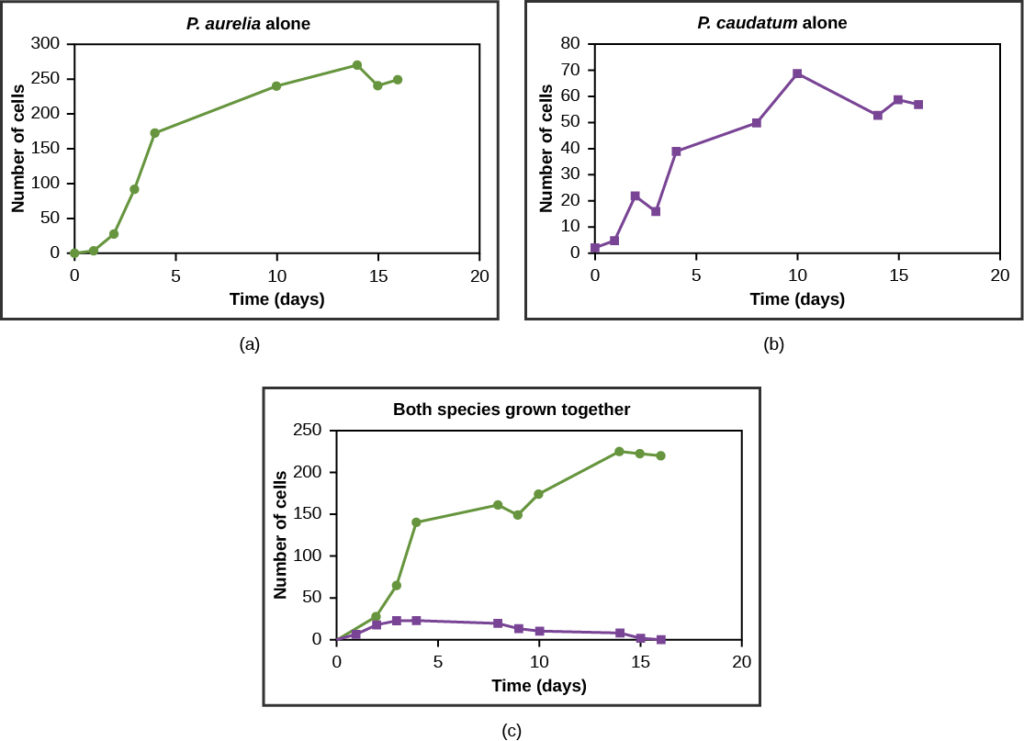
This exclusion may be avoided if a population evolves to make use of a different resource, a different area of the habitat, or feeds during a different time of day, called resource partitioning. The two organisms are then said to occupy different microniches. These organisms coexist by minimizing direct competition.
Resource Partitioning
Competitive exclusion may be avoided if one or both of the competing species evolves to use a different resource, occupy a different area of the habitat, or feed during a different time of day. The result of this kind of evolution is that two similar species use largely non-overlapping resources and thus have different microniches. This situation is called resource partitioning, and it helps the species coexist because there is less direct competition between them. These organisms coexist by minimizing direct competition.
The anole lizards found on the island of Puerto Rico are a good example of resource partitioning. In this group, natural selection has led to the evolution of different species that make use of different resources. The figure below shows resource partitioning among 11 species of anole lizards. Each species lives in its own preferred habitat, which is defined by the type and height of vegetation (trees, shrubs, cactus, etc.), sunlight, and moisture, among other factors.
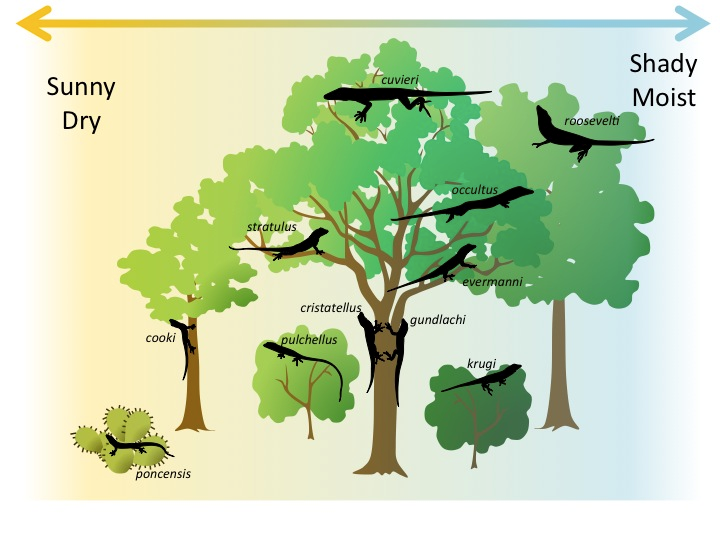
Watch this video to review competition and how populations share resources in a community:
Did I Get It?
Symbiosis
Symbiotic relationships, or symbioses (plural), are close interactions between individuals of different species over an extended period of time that impact the abundance and distribution of the associating populations. Most scientists accept this definition, but some restrict the term to only those species that are mutualists in a situation in which both individuals benefit from the interaction. In this discussion, the broader definition will be used.
Commensalism
A commensal relationship occurs when one species benefits from the close, prolonged interaction while the other neither benefits nor is harmed. Birds nesting in trees provide an example of a commensal relationship (Figure 9). The tree is not harmed by the presence of the nest among its branches. The nests are light and produce little strain on the structural integrity of the branch, and most of the leaves, which the tree uses to get energy by photosynthesis, are above the nest, so they are unaffected. The bird, on the other hand, benefits greatly. If the bird had to nest in the open, its eggs and young would be vulnerable to predators. Another example of a commensal relationship is the pilot fish and the shark. The pilot fish feed on the leftovers of the host’s meals, and the host is not affected in any way.

Mutualism
A second type of symbiotic relationship is called mutualism, where two species benefit from their interaction. Some scientists believe that these are the only true examples of symbiosis. For example, termites have a mutualistic relationship with protozoa that live in the insect’s gut (Figure 10a). The termite benefits from the ability of bacterial symbionts within the protozoa to digest cellulose. The termite itself cannot do this, and without the protozoa, it would not be able to obtain energy from its food (cellulose from the wood it chews and eats). The protozoa and the bacterial symbionts benefit by having a protective environment and a constant supply of food from the wood-chewing actions of the termite. Lichens have a mutualistic relationship between fungi and photosynthetic algae or bacteria (Figure 10b). As these symbionts grow together, the glucose produced by the algae provides nourishment for both organisms. In contrast, the physical structure of the lichen protects the algae from the elements and makes certain nutrients in the atmosphere more available to the algae.

One of the most remarkable examples of mutualism is between vascular plants and a type of fungi called mycorrhizae. The name mycorrhiza is derived from the Greek words myco meaning “fungus” and rhizo meaning “root.” In a mycorrhizal association, the fungi use their extensive network of hyphae (long, branching, filamentous structures that spread underground) and large surface area in contact with the soil to channel water and minerals from the soil into the plant. In exchange, the plant supplies the products of photosynthesis to fuel the metabolism of the fungus. Nearly 90 percent of all vascular plant species have mycorrhizal partners. A well-supported theory proposes that these fungi were instrumental in the evolution of the root system in plants and contributed to the overwhelming success of flowering plants. The plants benefited from the association because mycorrhizae allowed them to move into new habitats and allowed the increased uptake of nutrients, which gave them an enormous selective advantage over plants that did not establish symbiotic relationships.
Human life is only possible due to the action of microbes, both those in the environment and those species that call us home. Internally, they help us digest our food, produce crucial nutrients for us, protect us from pathogenic microbes, and help train our immune systems to function correctly. Each individual has a normal microbial flora (also known as a gut microbiota)—these terms simply refer to the collective of the bacteria living in each person’s stomach. When these bacteria counts change, it can cause digestive problems.
The bacteria that inhabit our skin and gastrointestinal tract do a host of good things for us. They protect us from pathogens, help us digest our food, and produce some of our vitamins and other nutrients. These activities have been known for a long time. More recently, scientists have gathered evidence that these bacteria may also help regulate our moods, influence our activity levels, and even help control weight by affecting our food choices and absorption patterns. The Human Microbiome Project has begun the process of cataloging our normal bacteria (and archaea) so we can better understand these functions.
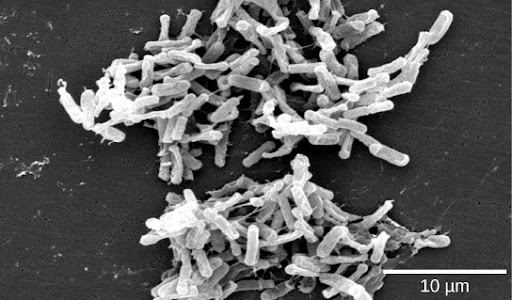
A particularly fascinating example of our normal flora relates to our digestive systems. People who take high doses of antibiotics tend to lose many of their normal gut bacteria, allowing a naturally antibiotic-resistant species called Clostridium difficile to overgrow and cause severe gastric problems, especially chronic diarrhea (Figure 11). Obviously, trying to treat this problem with antibiotics only makes it worse. However, it has been successfully treated by giving the patients fecal transplants from healthy donors to reestablish the normal intestinal microbial community. Clinical trials are underway to ensure the safety and effectiveness of this technique.
Scientists are also discovering that the absence of certain key microbes from our intestinal tract may set us up for a variety of problems. This seems to be particularly true regarding the appropriate functioning of the immune system. There are intriguing findings that suggest that the absence of these microbes is an important contributor to the development of allergies and some autoimmune disorders. Research is currently underway to test whether adding certain microbes to our internal ecosystem may help in the treatment of these problems as well as in treating some forms of autism.
Parasitism
A parasite is an organism that lives in or on another living organism and derives nutrients from it. In this relationship, the parasite benefits, but the host is harmed. The parasite usually weakens the host as it siphons resources the host would normally use to maintain itself. The parasite, however, is unlikely to kill the host, especially not quickly, because this would allow no time for the organism to complete its reproductive cycle by spreading to another host.
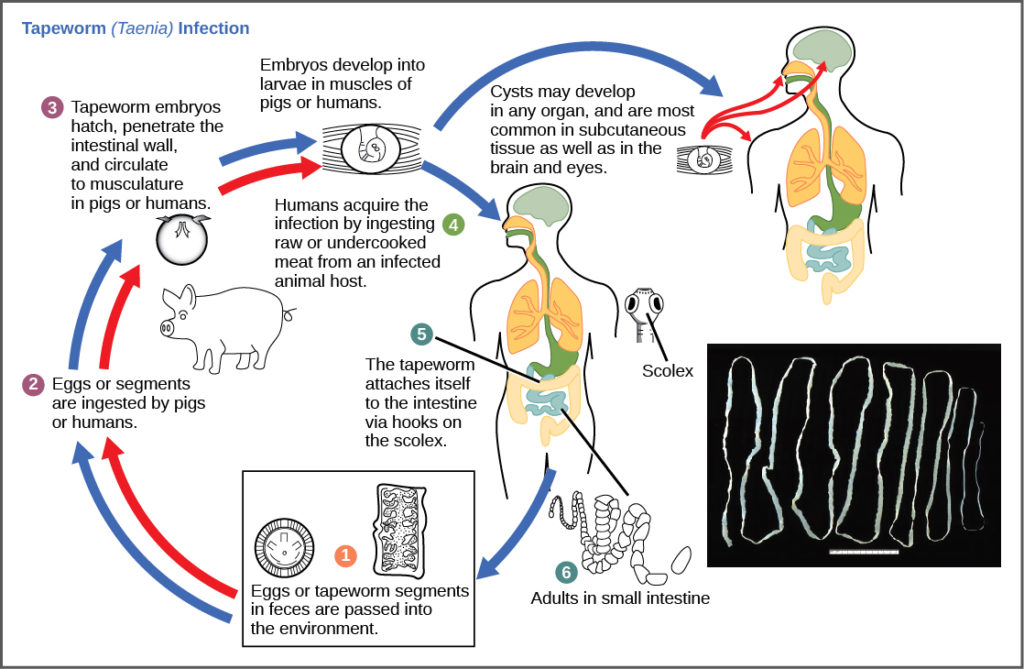
The reproductive cycles of parasites are often very complex, sometimes requiring more than one host species. A tapeworm is a parasite that causes disease in humans when contaminated, undercooked meat is consumed (Figure 12). The tapeworm can live inside the intestine of the host for several years, benefiting from the food the host is eating, and may grow to be over 50 ft long by adding segments. The parasite moves from species to species in a cycle, making two hosts necessary to complete its life cycle.
Another common parasite is Plasmodium falciparum, the protozoan cause of malaria, a significant disease in many parts of the world. Living in the human liver and red blood cells, the organism reproduces asexually in the gut of blood-feeding mosquitoes to complete its life cycle. Thus malaria is spread from human to human by mosquitoes, one of many arthropod-borne infectious diseases.
Did I Get It?
Scientist Spotlight: Lawrence David
Computational biologist Lawrence David studies the human microbiome. Research in his lab is focused on understanding how the microbial community in and on the human body behaves and changes over time and under varying conditions. Read about his fascinating yearlong study of his own gut microbiome in the Science News articles Lawrence David’s gut check gets personal and How one scientist’s gut microbes changed over a year, and check out the next video where David explains why he studies poop!
Characteristics of Communities
Communities are complex entities that can be characterized by their structure (the types and numbers of species present) and dynamics (how communities change over time). Understanding community structure and dynamics enables community ecologists to manage ecosystems more effectively.
Foundation Species
Foundation species are considered the “base” or “bedrock” of a community, having the greatest influence on its overall structure. They are usually the primary producers: organisms that bring most of the energy into the community. Kelp, or brown algae, is a foundation species, forming the basis of the kelp forests off the coast of California (Figure 13).

Foundation species may physically modify the environment to produce and maintain habitats that benefit the other organisms that use them. An example is the photosynthetic corals of the coral reef (Figure 14). Corals themselves are not photosynthetic but harbor symbionts within their body tissues (dinoflagellates called zooxanthellae) that perform photosynthesis; this is another example of a mutualism. The exoskeletons of living and dead coral make up most of the reef structure, which protects many other species from waves and ocean currents.

In the next video from TEDx Youth, high school student John Ye presents the impact of climate change on coral reefs, and the solutions that are being explored by scientists.
Biodiversity, Species Richness, and Relative Species Abundance
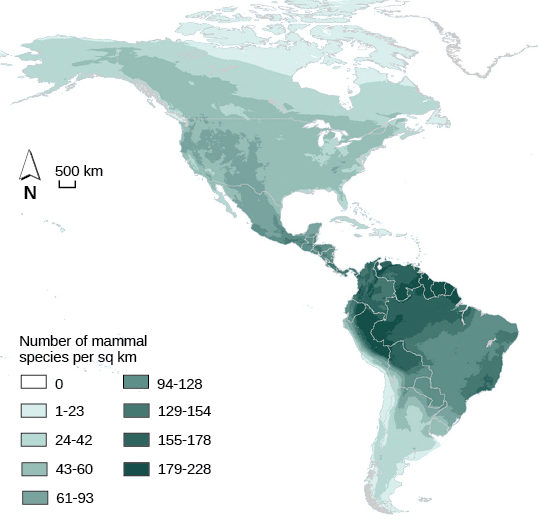
Biodiversity describes a community’s biological complexity: it is measured by the number of different species (species richness) in a particular area and their relative abundance (species evenness). The area in question could be a habitat, a biome, or the entire biosphere. Species richness is the term that is used to describe the number of species living in a habitat or biome. Species richness varies across the globe (Figure 15). One factor in determining species richness is latitude, with the greatest species richness occurring in ecosystems near the equator, which often have warmer temperatures, large amounts of rainfall, and low seasonality. The lowest species richness occurs near the poles, which are much colder, drier, and thus less conducive to life in Geologic time (time since glaciations). The predictability of climate or productivity is also an important factor. Other factors influence species richness as well. For example, the study of island biogeography attempts to explain the relatively high species richness found in certain isolated island chains, including the Galápagos Islands that inspired the young Darwin. Relative species abundance is the number of individuals in a species relative to the total number of individuals in all species within a habitat, ecosystem, or biome. Foundation species often have the highest relative abundance of species.
Keystone Species
A keystone species is one whose presence is key to maintaining biodiversity within an ecosystem and to upholding an ecological community’s structure. The intertidal sea star, Pisaster ochraceus, of the northwestern United States is a keystone species (Figure 16). Studies have shown that when this organism is removed from communities, populations of their natural prey (mussels) increase, completely altering the species composition and reducing biodiversity. Another keystone species is the banded tetra, a fish in tropical streams, which supplies nearly all of the phosphorus, a necessary inorganic nutrient, to the rest of the community. If these fish were to become extinct, the community would be greatly affected.

Scientist Spotlight: Ynés Mexía

When retracing the history of ecology, it is easy to come to a full stop at Charles Darwin’s On the Origin of Species. His theory of evolution by natural selection shook the scientific community to its core, and observations used to synthesize this theory were collected on a five-year voyage around the world. However, Darwin was not the only one to achieve an expedition of such a grand scale.
Ynés Mexía was a Mexican-American botanist and adventurer. Motivated by a newfound interest in science, Ynes Mexia enrolled in classes at UC Berkeley in 1921. She was 51 years old at the time. By the age of 55, she was engaged in a series of botanical expeditions in remote Alaska and South/Central America. Alongside a Stanford University botanist, Mexia collected 500 plant specimens on her first trip to Mexico. She went on to collect about 145,000 specimens over the next 13 years, 500 of which were new species. Charles Darwin collected a mere 500 specimens on his famed five-year voyage. In addition to the sheer number of Mexia’s samples, she was a natural scientist whose research contributed immensely to the modern classification of plants in North America.
Despite her impressive fieldwork, Mexia never received recognition comparable to that of her male counterparts. However, of the 500 plant species she discovered, 50 were named after her, as well as the entire genus Mexianthus (Figure 18).

Learn more about this trailblazing botanist in the Massive Science article Meet Ynes Mexia, late-blooming botanist whose adventures rivaled Darwin’s, in the UNLADYLIKE2020 profile of Ynes Mexia, and this episode of Celebrating Science from the Detroit Zoo!
CC Licensed Content, Shared Previously, Included in How Organisms Get Energy
- Biology 2e. Authors: Mary Ann Clark, Matthew Douglas and Jung Choi. Provided by: OpenStax CNX. Located at: Biology 2e. License: CC BY: Attribution 4.0.
- Biology for Majors II. Authors: Shelly Carter and Monisha Scott. Provided by: Lumen Learning. Located at: Biology for Majors II | Simple Book Production. License: CC BY: Attribution 4.0.
- Niches & competition. Provided by: Khan Academy. Located at: Niches & Competition (article) | Ecology | Khan Academy. License: CC BY-NC-SA
- Scientist Spotlight – Ynes Enriquetta Julietta Mexia – Ecology for All! Provided by: BioLibre Texts. Located at: 3.3: Scientist Spotlight – Ynes Enriquetta Julietta Mexia – Biology LibreTexts. License: CC BY-NC-SA

