Cancer requires multiple mutations
The mechanism by which a cell gains a growth advantage can vary widely from cancer to cancer. There are likely over a thousand human tumor suppressors and proto-oncogenes that can contribute to oncogenesis. This makes cancers genetically distinct from patient to patient and tumor to tumor. Even cancers of the same tissue can have a different combination of mutations.
In healthy cells, proto-oncogenes promote cell division, but only when conditions are right. When mutated, oncogenes cause cell division all the time (even when conditions are inappropriate). Rapidly dividing cells are more likely to accumulate additional mutations, since each round of replication is a chance to make a mistake. This rapid division of cells is described as genomic instability. If tumor suppressors are mutated, the mutation leads to a loss of cell cycle inhibition and/or a rapid accumulation of mutations, including additional oncogenic mutations that lead to overactive cell division.
In this respect, mutations in either a proto-oncogene or tumor suppressor can make it more likely to accumulate even more mutations.
Typically, mutations in both proto-oncogenes and tumor suppressors are needed for a cell to become cancerous – and usually mutations in multiple proto-oncogenes and multiple tumor suppressors. One estimate is that there are, on average, 5-6 mutations driving most cancers although there are notable exceptions with fewer mutations or far more.[1] Because of this, oncogenesis typically happens in stages, with each acquired mutation giving a cell (and its offspring) a slight growth advantage over surrounding healthy tissue.
An example of colon cancer is shown in Figure 18. Typically, each stage is associated with a slight growth advantage. So, in stage 1, the loss of APC tumor suppression confers a slight growth advantage to a mutant cell, leading to the growth of a polyp in the colon. In subsequent stages, the rapid growth increases the likelihood of acquiring more mutations. More mutations overall mean an increased chance an oncogene will be mutated in one cell of the polyp. This, in turn, makes the cell divide more often, making the cell (and its descendants) more likely to acquire additional mutations. The cell growth and genomic instability are therefore interconnected.
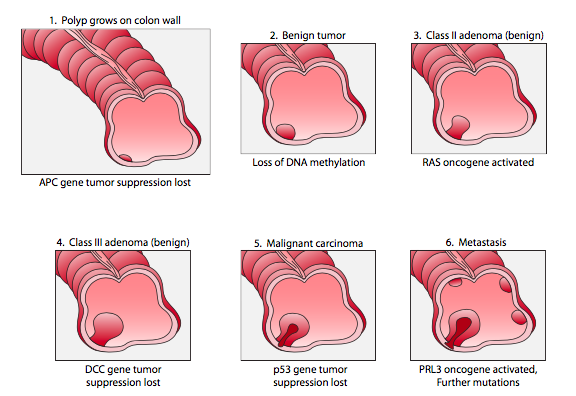
Because oncogenesis occurs in stages, the cells of a single tumor can be quite heterogeneous genetically, as illustrated in Figure 19. This can make it challenging for treatment, as not all cells of a tumor may respond equally to a drug regimen. As a cancer is treated, cells will continue to divide and acquire mutations, even as other cells are killed by a regiment. This can ultimately lead to a drug-resistant tumor, in much the same way as antibiotic misuse can lead to antibiotic resistance.
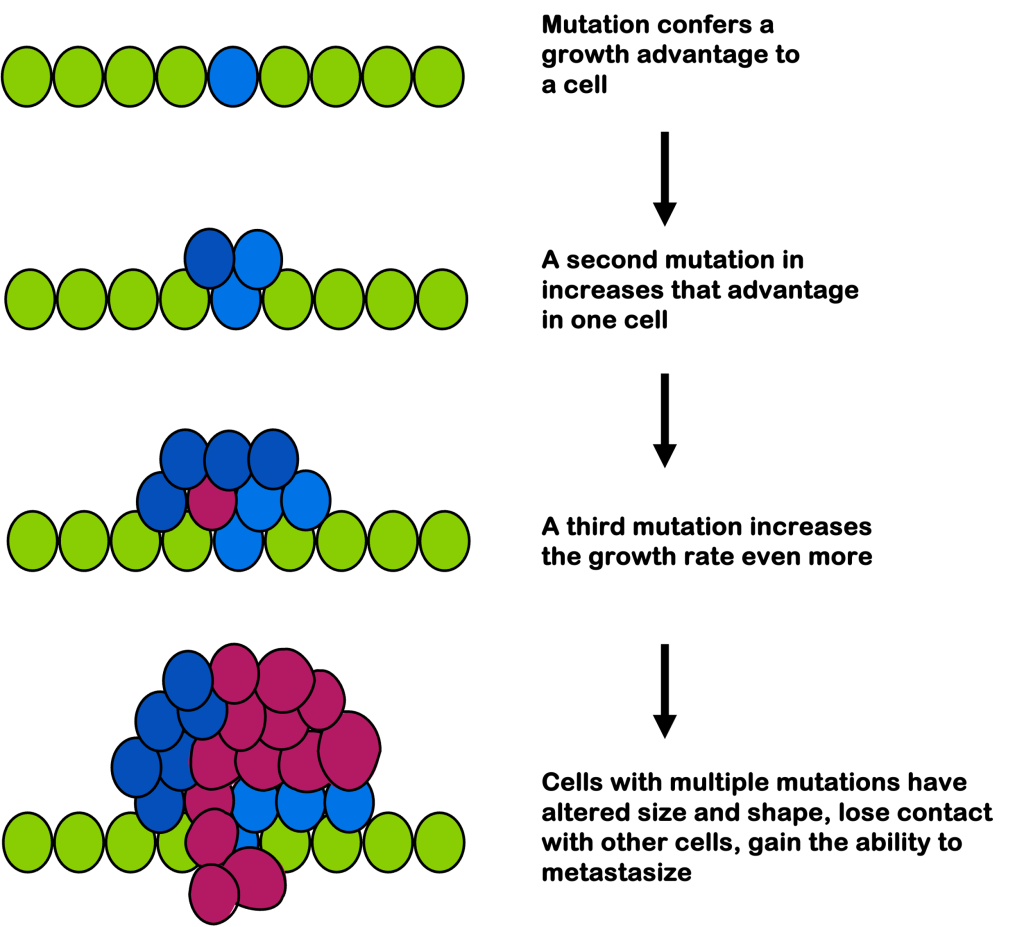
In later stages, cells may appear de-differentiated, as if they’ve “forgotten” the type of cell they were. They have altered size and shape compared to their surroundings. And they no longer stop growth on contact with surrounding cells: they can invade surrounding tissues.
As the tumor grows and additional mutations accumulate, most tumors acquire certain characteristics that distinguish them from their healthy surroundings. These are called the Hallmarks of Cancer, first proposed by Douglas Hanahan and Robert Weinberg in 2000, with updates in 2011 and 2022 as the physiology of cancer became better understood.
Among the original Hallmarks of Cancer:
- Cell division without growth signals
- Cell division in the presence of inhibitory signals
- Evading cell death
- Cell immortality (activation of telomerase, for example)
- Accessing or inducing vasculature (blood vessels)
- Metastasis
To these hallmarks were later added: avoiding immune detection, inflammation, genome instability, and deregulating cell metabolism. And most recently: epigenetic changes, microbiome influences, senescent cells, and reshaping cell fate (including de-differentiation or assuming a different cellular phenotype).
Because these hallmarks distinguish cancer cells from healthy tissue, they are attractive targets for cancer therapeutics. Cancer therapeutics are designed to kill cells with these characteristics.
Test Your Understanding
Media Attributions
- Figure 18 DNA Repair © Cells – Molecules and Mechanisms (Wong) is licensed under a CC BY (Attribution) license
- Figure 19 DNA Repair © Amanda Simons is licensed under a CC BY-SA (Attribution ShareAlike) license
- Knudson, A. G. Two genetic hits (more or less) to cancer. Nat. Rev. Cancer 1, 157–162 (2001). ↵

