Chemistry of replication and transcription
DNA replication and transcription are similar in chemistry. Both processes use nucleotide triphosphates, or NTPs, as building blocks to assemble a new polymer (Figure 3). And both replication and transcription use an enzyme called a polymerase to add nucleotides to the 3’ end of a growing polymer.
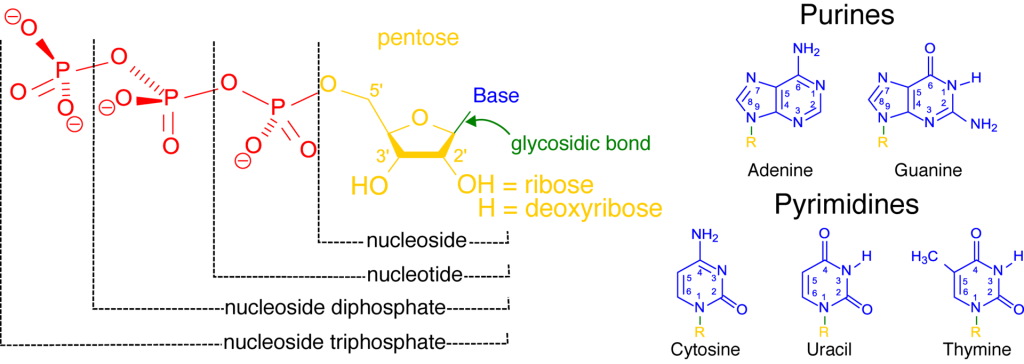
A nucleotide may include one, two, or three phosphates (red in Figure 3) linked to the 5’ carbon of a five-carbon sugar (yellow). The phosphate closest to the sugar is called the alpha phosphate, the middle one is the beta phosphate, and the one farthest from the sugar is the gamma phosphate.
As a reminder, DNA nucleotides use the sugar deoxyribose, with an OH group on the 2’ carbon. RNA nucleotides use the sugar ribose, with a hydrogen (H) on the 2’ sugar. A nitrogenous base (blue) is connected to the sugar via glycosidic bond. The structures of five bases are shown. DNA uses bases adenine, guanine, cytosine, and thymine, while RNA uses uracil instead of thymine. The nucleotides are named for the base they include, so the four DNA nucleotides are adenosine, guanosine, cytidine, thymidine, and uridine. The RNA nucleotides are often abbreviated ATP, CTP, GTP, and UTP. The DNA nucleotides are often abbreviated dATP, dCTP, dGTP, and dTTP.
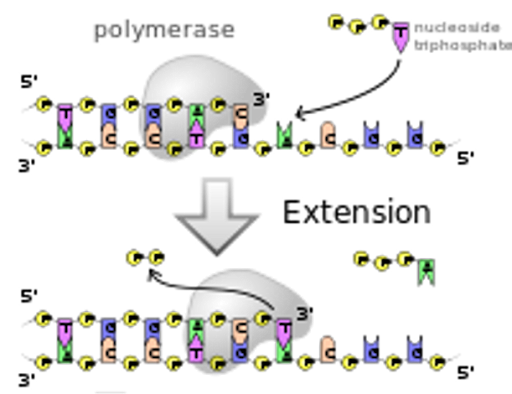
The general process of replication and transcription is shown in Figure 4. The bottom longer strand in the image is part of the original DNA molecule. The top strand is the newly synthesized strand, which forms complementary base pairs with the template. New nucleotides are added to the 3’ end of the daughter strand in a reaction catalyzed by a polymerase enzyme. Because of this, we say that both replication and transcription proceed in a 5’ to 3’ manner.
Note that the template and daughter strands are antiparallel in orientation, with the 5’ end of the daughter aligning with the 3’ end of the template. Polymerases therefore move toward the 5’ end of the template.
Adenosine triphosphate (used for RNA) is the same ATP molecule that powers cellular reactions. dATP is almost identical – it is just missing the 2’ OH group. Just like the breakdown of ATP powers cellular reactions, the high-energy bonds connecting the phosphates of all the dNTPs and NTPs serve as an energy store to power DNA and RNA synthesis. When an incoming nucleotide is added to the growing strand, the high-energy bond connecting the alpha and beta phosphates is broken. The energy released powers the formation of a phosphodiester bond between the incoming nucleotide and the growing chain.
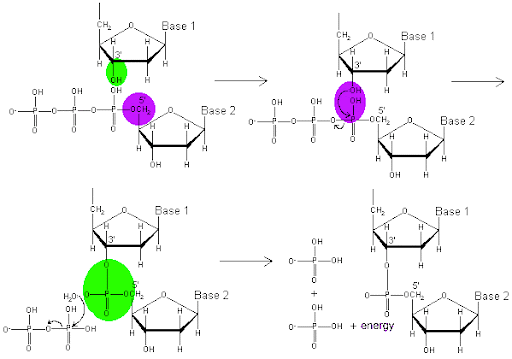
The mechanism of the reaction is shown in Figure 5. The green OH group represents the 3’ end of a newly synthesized daughter strand. The pink highlights the 5’ carbon of the incoming nucleotide triphosphate, from which extend three phosphate groups. The formation of the phosphodiester bond between the 3’OH and the phosphorus closest to the 5’ carbon results in the release of two of the phosphates from the nucleotide. The release of the diphosphate, and the subsequent separation of the two released phosphate groups, releases enough energy to drive the formation of the phosphodiester bond. Note that, although DNA is shown in Figure 5, the only difference for RNA would be an OH group at the 2’ position of each nucleotides.
Of course, there are significant differences between replication and transcription. We will start with replication, which requires that both strands of DNA are read simultaneously to create two new complementary strands, eventually resulting in a complete and nearly perfect copy of an entire organismal genome. Much of what we know about replication comes from studying prokaryotes, in particular the model organism E. coli. The next sections focus on replication in prokaryotes, with notes about the differences in eukaryotes.
Test Your Understanding
Media Attributions
- Structure of a nucleotide © Wikipedia is licensed under a CC0 (Creative Commons Zero) license
- The chemistry of DNA and RNA synthesis © Wikipedia is licensed under a CC0 (Creative Commons Zero) license
- DNA replication © Ed Vitz, John W. Moore, Justin Shorb, Xavier Prat-Resina, Tim Wendorff, & Adam Hahn is licensed under a CC BY-SA (Attribution ShareAlike) license
- Chemical structure © Wikipedia is licensed under a CC0 (Creative Commons Zero) license

