Familial vs sporadic cancers
Although cancer is a genetic disease, it is not inherited. A person who has cancer will not pass that tumor to their offspring because it arose from somatic mutations. But there are examples where a family may have a higher-than-expected incidence of cancer. These are called familial cancers, in contrast to sporadic cancers that occur in people with no family history of the disease.
Familial incidence of cancer occurs because family members share a disease-associated allele in a tumor suppressor gene. An important distinction is that the cancer itself is not inherited from a parent; rather, the susceptibility to disease is inherited.
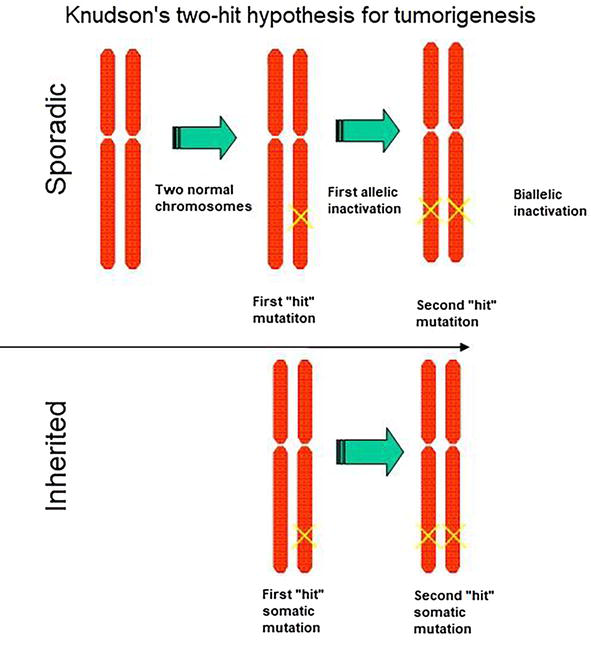
For most people to develop cancer, they need two mutations, or “hits,” to occur in a single cell: one in each allele. This is extremely rare, although the longer one lives, the more likely it is to happen just because all organisms accumulate mutations throughout their lifespan. For those who inherit a disease-associated variant, though, every cell in their body already has a head start. If only one cell acquires a mutation in the second allele, that cell will become cancerous. This is called the “two-hit hypothesis” for how cancer develops. (In practice, for most cancers, it’s more like 5-6 hits, as discussed in the previous section!)
Tumor suppressor Rb provides a good example of a two-hit cancer. Most incidences of retinoblastoma are sporadic, and patients only have unilateral tumors (meaning in one eye). But there are examples of familial retinoblastoma. Patients with a family history of the disease typically showed signs of the disease at a younger age, and they often developed tumors in both eyes. This is because the retinal cells of those patients only needed to acquire one more mutation to begin oncogenesis. Children who inherit a germline disease-associated variant of Rb have a 90% chance of developing retinoblastoma in one or both eyes. They also have an increased chance of developing other cancers later in life. The incomplete penetrance is due to the random nature of that second mutation.
Other familial cancers are similar. People with one germline disease-associated variant of p53 have Li-Fraumeni syndrome. People with Li-Fraumeni syndrome have nearly a 90% chance of developing at least one cancer throughout their lifetime.
Cisgender females who inherit one disease-associated allele of the tumor suppressors BRCA1 or BRCA2 have a 70% chance of developing breast cancer. Or, in other words, they have a 70% chance of losing BRCA1/2 function in the second allele. Note that although certain variants in BRCA1 and BRCA2 are associated with increased breast cancer risk in cisgender men, there is not currently enough data to calculate the risk for transgender individuals using gender-affirming hormones who have BRCA1/2 variants.[1][2]
The gene conversion mentioned in the double-strand break repair section sometimes plays a role in whether an individual gets cancer. The healthy cells of a patient who has inherited a cancer-associated allele will be heterozygous. But often, we see a loss of heterozygosity in tumor cells: the tumor cells are homozygous for the mutant allele. This is not just the acquisition of a new mutation since both alleles are the same. Instead, it is an example of improper homology-directed repair.
There are no known familial cancers associated with proto-oncogenes. Inheritance of even one germline mutation in a proto-oncogene is presumably lethal at early embryonic stages since the embryo cannot properly regulate cell division.

