Penetrance and Expressivity
Sometimes, the phenotype of an organism may not match exactly to what is expected from their genotype. This can happen when a phenotype/trait is multifactorial. This means what it sounds like: multiple factors are influencing a trait, not just a single gene. This can mean that several different genes contribute to a phenotype, or it might mean that the environment might affect the phenotype.
However, because geneticists are often only tracking one gene at a time, sometimes it’s not (yet) known why the genotype does not always yield the expected phenotype. Geneticists have vocabulary that describes the relationship between alleles and phenotype, even without considering the mechanism for the relationship.
Penetrance and expressivity are related concepts that describe the relationship between genotype and phenotype, when additional (known or unknown) factors affect the phenotype.
Penetrance
When an allele controls a phenotype, but that phenotype does not always appear, this is called incomplete penetrance. Penetrance is often described as a percentage. An allele that always correlates with phenotype – like blood type in humans or petal color in snapdragons, and most of the alleles we’ve described previously – is described as 100% penetrant. But many traits show less than 100% penetrance: only a subset of individuals with the genotype display the phenotype.
In many cases, the mechanism for the incomplete penetrance may not be known: geneticists might just know that the phenotype does not always match what’s expected by the genotype. However, sometimes the mechanism of incomplete penetrance is known.
For example, certain variations in the human BRCA1 gene predispose patients to breast cancer. People heterozygous for these alleles have a very high occurrence of breast cancer: they have about a 70% chance of developing breast cancer in their lifetime. But it’s not 100%. The phenotype is thus 70% penetrant.
In this case, the incomplete penetrance is due to random chance: for a cancer to develop, one (or more) cells within a heterozygous patient’s body must sustain an additional DNA mutation in the second, healthy BRCA1 allele. This is a relatively rare occurrence, but given the number of cells in the human body and enough time, about 70% of heterozygous people do acquire that second mutation through random chance. Environmental factors can also play a role: Exposure to radiation or DNA damaging agents can increase the likelihood of mutation. Many other cancer-associated mutations are also incompletely penetrant for the same reason.
A second example of incomplete penetrance are the health problems and intellectual disabilities that can result from the metabolic disease phenylketonuria. Phenylketonuria (PKU) is caused by a defect in a gene responsible for metabolizing the amino acid phenylalanine, which is part of a normal diet. Phenylalanine is even found in foods you might not expect: it is a component of the artificial sweetener aspartame.
Exposure to phenylalanine during infancy causes the intellectual disability characteristic of the disorder. However, most infants in the US are tested for this (and other) metabolic disorders at birth, and individuals with PKU can avoid most symptoms through a diet low in phenylalanine. Diet therefore prevents the symptoms of PKU and makes the phenotype incompletely penetrant[1]. In the United States, if you look at the nutritional labels for foods and beverages containing aspartame, you will see a statement that says, “Phenylketonurics: contains phenylalanine”, which is a warning to people who need to adhere to a low-phenylalanine diet.
Expressivity

Variable expressivity, on the other hand, describes a phenotype that varies in the degree to which it is expressed.
An example of variable expressivity in dogs is the coat color seen in yellow Labrador retrievers (and other dogs, too!). Yellow Labs are all homozygous for the recessive allele of the E locus (the MC1R gene), which prevents the production of the pigment eumelanin in the hairs of the fur. But some “yellow” Labrador retrievers are more cream colored, while others are more golden with reddish undertones (Figure 6). This variation is due to differences in other genes that modify coat color in dogs.
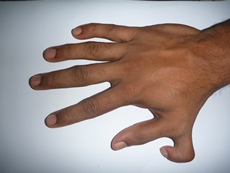
An example of variable expressivity in humans is polydactyly: the presence of extra digits on the feet or hands (Figure 7). Although alleles of several different genes can cause this phenotype, most are dominant, with variable expressivity. The phenotype is variable among patients with a polydactyly-associated allele, ranging from an extra underdeveloped partial finger or toe to multiple additional digits on both hands and feet. The phenotype can even vary for an individual, with left and right hands or feet showing differences.
Incomplete penetrance and variable expressivity can both be caused by additional genetic factors (as in the yellow Labradors), environmental conditions (as in the diet of patients with PKU), and even randomness (as in the acquisition of a somatic mutation causing breast cancer in patients homozygous for a mutant BRCA1 allele). And more than one of these can contribute! For example, the BRCA1 breast cancer phenotype is affected both by randomness and by environmental factors: exposure to DNA damaging chemicals or radiation increases the likelihood of acquiring a somatic mutation.
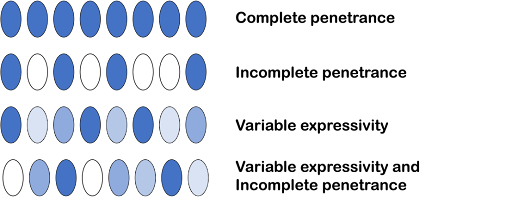
A trait can even be both incompletely penetrant and variably expressive. This is illustrated in Figure 8, where some individuals with a particular genotype do not have the trait at all, some have the trait to a small extent, and some have the trait to a large extent.
Media Attributions
- Labrador Retrievers © Wikipedia is licensed under a CC0 (Creative Commons Zero) license
- Polydactyly sreeakhil © Wikipedia is licensed under a CC0 (Creative Commons Zero) license
- Penetrance © Amanda Simons is licensed under a CC0 (Creative Commons Zero) license
- Cooper, D. N., Krawczak, M., Polychronakos, C., Tyler-Smith, C. & Kehrer-Sawatzki, H. Where genotype is not predictive of phenotype: towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum Genet 132, 1077–1130 (2013). ↵

