The chemical nature of nucleic acids
Nucleic acids serve as a biological information storage and retrieval system. The two main types of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA and RNA are called nucleic acids because they were identified in the nucleus of cells, and they function as acids: part of the molecule gives up a proton (H+) and is negatively charged at physiological pH.
DNA is the information storage system: it is the genetic material in all living organisms, ranging from single-celled bacteria to multicellular mammals. RNA primarily serves in the retrieval of information from DNA. RNA has several different functions in cells, most of which are involved in protein synthesis. For example, messenger RNA (mRNA) is used an intermediate between DNA and protein synthesis. Other types of RNA—like rRNA, tRNA, and microRNA—are involved in the process of protein synthesis and its regulation. RNA is also used by some viruses as genetic material: although viruses are not living, viruses do share many biochemical characteristics with living things.
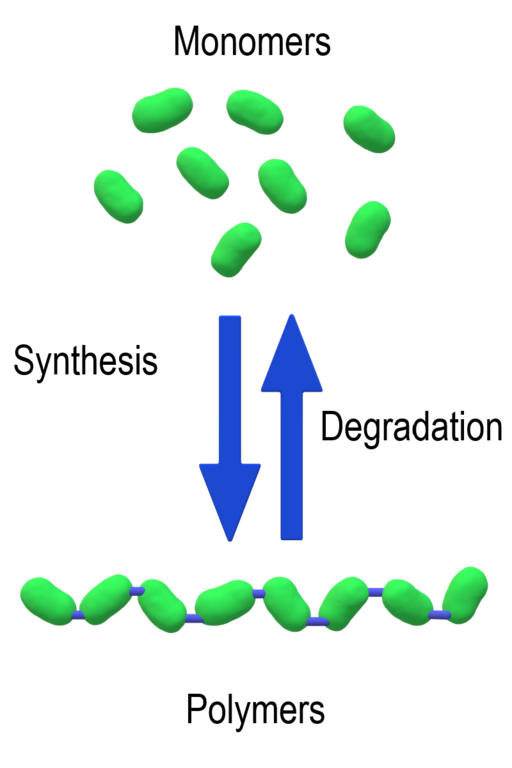
Both DNA and RNA are polymers. The prefix “poly” means “many”, and the word “polymer” is used to describe any molecule that is composed of a long chain of smaller building blocks. The individual subunits of any polymer are called “monomers”. This is illustrated in Figure 1.
The monomers of DNA and RNA are called nucleotides. The generic structure of a nucleotide is shown in Figure 2. Three components comprise each nucleotide: a nitrogenous base, a pentose (five-carbon) sugar, and one or more phosphate group (one phosphate group is shown in the nucleotide in Figure 2). The nucleotides are linked together one after another to form a polynucleotide, and it is the polynucleotide that is referred to as DNA or RNA.
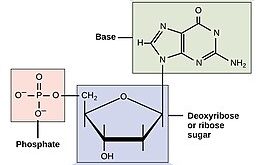
Nucleic acids are, in fact, acids. Remember from chemistry that an acid is a molecule that can give up a proton (H+). Under acidic conditions, the oxygens of the phosphate are protonated (meaning they are bound to hydrogen to form -OH). Under physiological pH (around pH 7), the oxygens of the phosphate give up their protons to become negatively charged. You can see the negative charges on the oxygens of the pink-highlighted phosphate group in Figure 2. The polymers DNA and RNA are likewise negatively charged in the cell.
Please note: the structures shown in these images use an organic chemistry shorthand, where the “C’s” representing carbon are not shown. Instead, if you see a “corner” in a structure with no atom indicated, you can assume there is a carbon at that position. Hydrogen atoms attached to carbons are also typically not shown unless they are specifically under discussion.
The nitrogenous base part of the nucleotide is highlighted in green. The nitrogenous bases contain nitrogen (thus giving them their name). They are bases because the nitrogen-containing groups (eg -NH2) have a lone pair of electrons and can act as electron-pair donors. If the pH were decreased, those groups would accept a proton and become positively charged (-NH3+). At physiological pH, though, the nitrogenous bases are uncharged.
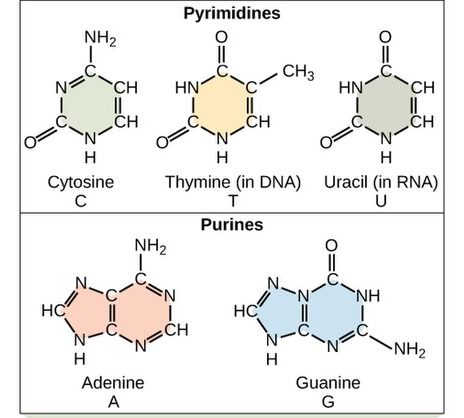
Each nucleotide in DNA contains one of four possible nitrogenous bases: adenine (A), guanine (G), cytosine (C), and thymine (T). RNA does not typically contain thymine. Instead, RNA contains the very similar base uracil, along with adenine, guanine, and cytosine. Uracil and thymine differ only by an extra methyl (-CH3) group. The structures of these five bases are shown in Figure 3.
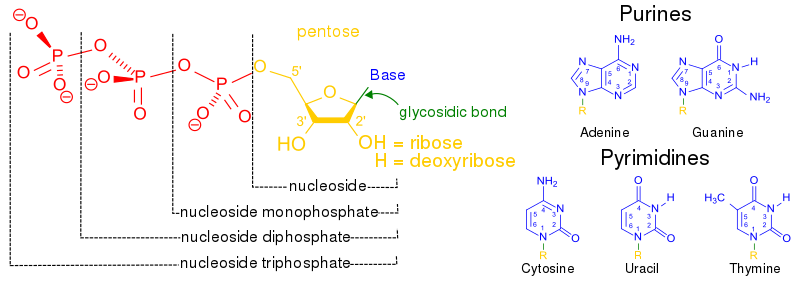
Adenine and guanine are classified as purines. The purine’s general structure is two carbon-nitrogen rings. Scientists classify cytosine, thymine, and uracil as pyrimidines, which have a single carbon-nitrogen ring as their general structure (Figure 2). Each of these basic carbon-nitrogen rings has different functional groups attached to it, which differentiate the bases from one another. In molecular biology shorthand, we know the nitrogenous bases by their symbols A, T, G, C, and U. DNA contains A, T, G, and C; whereas RNA contains A, U, G, and C.
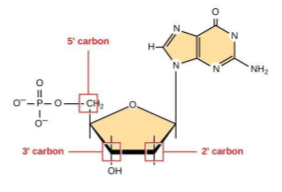
DNA and RNA nucleotides contain different sugars. The sugar in DNA is deoxyribose, and in RNA, the sugar is ribose. The difference between the sugars is the presence of the hydroxyl (-OH) group on the ribose’s second carbon and hydrogen (-H) on the deoxyribose’s second carbon. (Figure 4, bottom left).
To give scientists a way to talk about specific parts of the molecule, the carbon atoms of the sugar molecule are numbered as 1′, 2′, 3′, 4′, and 5′ (1′ is read as “one prime”). The prime distinguishes these atoms from those in the base, which are numbered without using a prime notation. As shown in Figure 4, the carbons are numbered, starting with the carbon attached to the base, which is 1’. Numbering continues around the ring in order, ending with 5’ as the carbon linked to the phosphate. Note that only the 2’, 3’, and 5’ carbons are highlighted in this image.
Given this numbering scheme, deoxyribose has an -H at the 2’ position, while ribose has an -OH.
Nucleotides are named in a way that indicates which base (adenine, guanine, thymine, cytosine, or uracil), which sugar, and how many phosphates are part of the molecule. Together, a base and a sugar make up a smaller unit that is called a nucleoside. Nucleosides containing adenine, guanine, cytosine, and thymine are called adenosine, guanosine, cytidine, and thymidine, respectively.
An RNA nucleotide containing adenine is adenosine 5’monophosphate, adenosine 5’diphosphate, or adenosine 5’triphosphase, depending on how many phosphate groups are part of the structure. These are often abbreviated AMP, ADP, and ATP for short. A DNA nucleotide containing adenine and three phosphate groups would be called 2’deoxyadenosine 5’triphosphate (or dATP for short). These structures are illustrated in Figure 5. The phosphate groups are named alpha (α), beta (β), and gamma (γ) for their proximity to the sugar, with the alpha phosphate closest to the sugar and the gamma farthest.
The nucleotides combine with each other via phosphodiester bonds. The phosphate residue attached to the 5′ carbon of the sugar of one nucleotide forms a second ester linkage with the hydroxyl group of the 3′ carbon of the sugar of the next nucleotide in a dehydration reaction that forms a 5′-3′ phosphodiester bond. Once the nucleotides are linked, the dinucleotide will have a 5’ phosphate at one end, and a 3’-OH group at the other. A polynucleotide may have thousands of such phosphodiester linkages. Thus, in a polynucleotide, one end of the chain has a free 5′ phosphate, and the other end has a free 3′-OH. These are called the 5′ and 3′ ends of the chain.
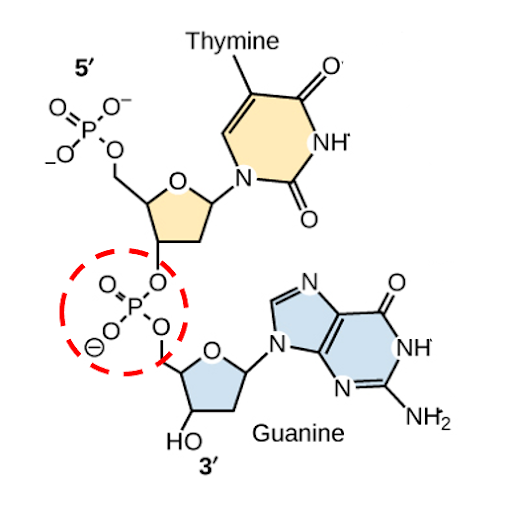
In Figure 6, a dinucleotide of thymine and guanine are shown, with the 5’ end of the short dinucleotide chain at the top of the image, the 3’ end of the dinucleotide at the bottom, and the phosphodiester bond circled in red. On the left side of the structure, you can see phosphate-sugar-phosphate-sugar alternating from top to bottom, with the bases extending to the right from the sugars. This is called the phosphate-sugar backbone.
Media Attributions
- Synthesis and Degradation © Christinelmiller is licensed under a CC BY-SA (Attribution ShareAlike) license
- Structure of a nucleotide © CNX OpenStax, CC BY 4.0 , via Wikimedia Commons is licensed under a CC BY-SA (Attribution ShareAlike) license
- Nitrogenous © Wikipedia is licensed under a CC BY-SA (Attribution ShareAlike) license
- Sugar structure © Adapted from OpenStax Biology is licensed under a CC BY-SA (Attribution ShareAlike) license
- Nucleotide vs nucleoside structure © Wikimedia Commons is licensed under a Public Domain license
- Dinucleotide of thymine and guanine © OpenStax Biology is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license

